News
Lady Prelox® Found to Naturally Improve Emotional, Physical Health and Sexual Function in Peri-Menopausal Women
Study shows Lady Prelox® may improve sexual function, and relieve climacteric symptoms in peri-menopausal women.
GENEVA, Switzerland – According to a double-blind, placedo-controlled study published in the Journal of Women’s Health Care, Lady Prelox® – designated as PACR: Pycnogenol® (French Pine Bark Extract) , L-Arginine, L-Citrulline, Rosvita® (Rose Hip Extract) – significantly improved sexual function as measured by 60% after 1 month and by 73% after 2 months treatment compared to baseline, whereas increase in the placebo group was only 40 and 46%. In addition, Lady Prelox® relieved climacteric symptoms evaluated both by the Kupperman’s index and Women’s Health Questionnaire (WHQ). The efficacy of Lady Prelox® was significantly superior compared to placebo. No unwanted effects were reported. Sexual function was evaluated by the Female Sexual Function Index (FSFI), which has been established and validated and nowadays represents the standard tool for assessment of women’s sexual function.
This clinical study, conducted at the Department of Obstetrics and Gynecology of the Medical University of Sofia, included 80 peri-menopausal women, aged 40 to 50 years, with moderate sexual function problems. They were supplemented with 4 tablets of Lady Prelox® a day for a period of 8 weeks.
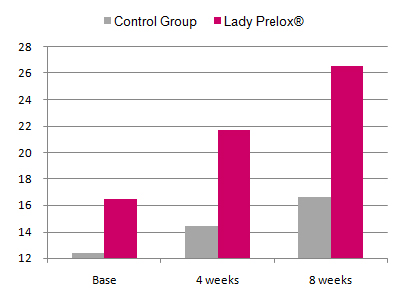
Effect of treatment on the women’s sexual function:
In placebo-treated women the total FSFI score non-significantly increased from 12.4 to 14.4 after 4 weeks and to 16.6 after 8 weeks. In the Lady Prelox® group the FSFI score at baseline was moderately higher than in the placebo group (16.5) and increased to 21.7 and 26.5 after 4 and 8 weeks, respectively. The scores for Lady Prelox® after 4 and 8 weeks of treatment were statistically significantly higher than in the placebo group.
A detailed examination of the different domains of the FSFI suggests that Lady Prelox® significantly improves “desire”, “arousal”, “lubrication”, “orgasm”, “satisfaction” and “pain”. Comparatively, the domains “orgasm” and “satisfaction” were found to improve most prominently.
Effect of treatment on climacteric symptoms:
Climacteric symptoms were found to significantly improve with Lady Prelox®, after 4 and 8 weeks of treatment. A series of symptoms was found to improve significantly already after 4 weeks as compared to the group of women taking placebo: hot flashes, night sweats, sleep problems, irritability, depressed mood and mental focus. These improvements were maintained until completion of the trial after 8 weeks.
All symptoms except “depressions” of the “Women Health Questionnaire” were significantly less pronounced than in the placebo group. Symptoms responding particularly well to Lady Prelox® were related to cognition (mental focus) and “anxiety/fears”. Interestingly, the symptom most influenced by the administration of placebo, was the self-perceived attraction.
Safety evaluations suggest that Lady Prelox® and placebo were well tolerated and none of the subjects experienced side-effects. Blood laboratory testing confirmed the safety of the product. A small yet statistical significant lowering of systolic and diastolic blood pressure was noticed with Lady Prelox® after eight weeks. Blood pressure, BMI, lipid profile and TAC significantly improved in the group receiving Lady Prelox®.
Conclusion
This study points to a very promising general improvement of well-being for women during the difficult transitional menopausal period. The simultaneous improvement of both sexual as well as climacteric symptoms warrants further investigation also for identification of long term benefits.
About Lady Prelox®
Lady Prelox® is a dietary supplement. Its patented formula is the result of years of scientific research. It contains only high quality premium ingredients which help with sexual desire, comfort and pleasure. Lady Prelox® has been clinically tested on more than 200 women and shown to help with female sexual pleasure, desire and comfort, measured using the Female Sexual Function Index – a tool used to record female sexual functions. Lady Prelox® is the first product of its kind to have undergone stringent both single- and double-blind, placebo controlled trials. For more information, visit www.ladyprelox.com
About Horphag Research
Horphag Research Ltd. is the exclusive worldwide supplier of Pycnogenol® (pic-noj-en-all) brand French maritime pine bark extract, Rosvita® rose hip extract, as well as proprietary formulations as Prelox® and Lady Prelox®. Pycnogenol®, Rosvita®, Prelox® and Lady Prelox® are registered trademarks of Horphag Research Ltd. Horphag Research has been awarded the Frost & Sullivan Excellence in Research Award, Nutraceutical Business & Technology Safety & Quality Award, SupplySide West Scientific Excellence Award and The American Botanical Council’s Tyler Research Award.